 Valtrex Tablets - NPS MedicineWise
Valtrex Tablets - NPS MedicineWiseCopyright © 2018 by RxList Inc. RxList does not provide medical advice, diagnosis or treatment. .Valtrex valtrex (hydrochloride valaciclovir) Find the lowest prices in Valtrex What is Valtrex and how is it used? What is Valtrex and how is it used? Valtrex is a prescription antiviral medication used to treat symptoms of , herpes zoster (pies) and zoster (Chickenpox). Valtrex can be used alone or with other medicines. Valtrex is an antiviral medication. What are the possible side effects of Valtrex? Valtrex may cause serious side effects, including: Get medical help right away, if you have any of the symptoms mentioned above. The most common side effects of Valtrex include: Get medical help right away, if you have any of the symptoms mentioned above. These are not all possible side effects of Valtrex. For more information, ask your doctor or pharmacist. Call your doctor to advise you about side effects. You may report side effects to FDA at 1-800-FDA-1088.DESCRIPTIONVALTREX (valacyclovir hydrochloride) is the chloride salt of the L-valyl ester of the medication. VALTREX Caplets are for oral administration. Each caplet contains chlorhydrate valaciclovir equivalent to 500 mg or 1 valaciclovir and inactive ingredients carnauba wax, colloidal silicon dioxide, crospovidone, FD pestC Blue No. 2 Lake, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polysorbate 80, povidone, and titanium dioxide. Blue and coated caplets are printed with edible white ink. The chemical name of chlorhydrate valaciclovir is L-, 2-[(2-amino-1,6-dihydro-6-oxo-9H-purin-9-yl)metoxi]etile ester, monohydrochloride. It has the following structural formula: Valacyclovir chlorhydrate is a white powder with the molecular formula C13H20N6O4•HCl and a molecular weight of 360.80. The maximum solubility in the water at 25°C is 174 mg/mL. The pkas for valaciclovir chloride are 1.90, 7.47 and 9.43. INDICATIONS Adult patientsVALTREX is indicated for the treatment of cold sores (herpes labialis). The effectiveness of VALTREX initiated after the development of clinical signs of a cold ulcer (e.g. papula, vesicle or ulcer) has not been established. The initial EpisodeVALTREX is indicated for the treatment of the initial episode of genital herpes in immunocompetent adults. The effectiveness of VALTREX treatment when more than 72 hours are started after signs and symptoms have not been established. Recurrent EpisodesVALTREX is indicated for the treatment of recurrent episodes of genital herpes in immunocompetent adults. The efficacy of VALTREX treatment when it starts more than 24 hours after signs and symptoms have not been established. Therapy of the ASSTRAEX is indicated for chronic suppressive therapy of recurrent episodes of genital herpes in immunocompetent adults and HIV-1. VALTREX's effectiveness and safety for the suppression of genital herpes beyond 1 year in immunocompetent patients and beyond 6 months in patients infected with HIV-1 have not been established. TransmissionVALTREX reduction is indicated for the reduction of the transmission of genital herpes in immunocompetent adults. The effectiveness of VALTREX for reducing the transmission of genital herpes beyond 8 months in discordant couples has not been established. VALTREX effectiveness has not been established for reducing the transmission of genital herpes in individuals with multiple non-heterosexual couples and couples. Safer sexual practices should be used with suppressive therapy (see current disease control and prevention centers [CDC] Guidelines for the Treatment of Sexually Communicable Diseases). VALTREX is indicated for the treatment of zoster herpes (pieces) in immunocompetent adults. The effectiveness of VALTREX when it began more than 72 hours after the start of the VALTREX eruption and the effectiveness and safety for the treatment of herpes dissected zoster have not been established. Pediatric patientsVALTREX is indicated for the treatment of cold sores (herpes labialis) in pediatric patients older or equal to 12 years. The effectiveness of VALTREX initiated after the development of clinical signs of a cold ulcer (e.g. papula, vesicle or ulcer) has not been established. VALTREX is indicated for the treatment of chickenpox in pediatric patients with immunocompetent 2 to less than 18 years. Based on clinical trial effectiveness data with oral acyclovir, VALTREX treatment should be started within 24 hours of the start of the eruption [see Clinical Studies]. Clinical studies Limitations of use VALTREX efficiency and safety have not been established in:QUESTIONDOSAGE AND ADMINISTRATIONExtemporary Suspension Preparation Oral Adult Dosage Recommendations The recommended dose of VALTREX for the treatment of cold sores is 2 grams twice a day for 1 day separated 12 hours. Therapy should start at the first symptom of a cold sore (e.g., tingling, itching, or burning). Initial Episode The recommended dose of VALTREX for the treatment of initial genital herpes is 1 gram twice a day for 10 days. The therapy was more effective when given within 48 hours of the appearance of signs and symptoms. Recurrent Episodes The recommended dose of VALTREX for the treatment of recurrent genital herpes is 500 mg twice a day for 3 days. Start treatment in the first sign or symptom of an episode. Surprising The recommended dose of VALTREX for chronic suppressive therapy of recurrent genital herpes is 1 gram once a day in patients with normal immune function. In patients with a history of 9 or less recurrence a year, an alternative dose is 500 mg once a day. In patients infected with a CD4+ with a number of cells above or equal to 100 cells/mm3, the recommended dose of VALTREX for chronic suppressive therapy of recurrent genital herpes is 500 mg twice a day. Transmission reduction The recommended dose of VALTREX for reducing the transmission of genital herpes in patients with a history of 9 or less recurrences a year is 500 mg once a day for the source partner. The recommended dose of VALTREX for herpes zoster treatment is 1 gram 3 times a day for 7 days. Therapy should start at the first sign or symptom of herpes zoster and is more effective when it starts within 48 hours of the eruption. Pediatric dosing recommendations The recommended dose of VALTREX for the treatment of cold sores in pediatric patients older or equal to 12 years is 2 grams twice a day for 1 day apart 12 hours. Therapy should start at the first symptom of a cold sore (e.g., tingling, itching, or burning). The recommended dose of VALTREX for the treatment of chickenpox in pediatric patients with 2-to-under 18-year-old immunocompetent is 20 mg/kg administered 3 times a day for 5 days. The total dose should not exceed 1 gram 3 times a day. Therapy should be started at the first sign or symptom [see Use in Specific Populations, Clinical PHARMACOLOGY, Clinical Studies]. Use in specific populations PHARMACOLOGYClinical StudiesExtemporaneous Preparation of 500 mg Oral Suspension Tablets, Cherry and Suspension Structured Vehicle USP-NF (SSV). The Valacyclovir oral suspension (25 mg/mL or 50 mg/mL) should be prepared in a pile of 100 ml.* The amount of cherry flavor added is how the cherry flavor suppliers instructed it. Patients with kidney disabilitiesThe treatment recommendations for adults with reduced kidney function are provided in Table 1 [see Use in specific populations, PHARMACOLOGY]. No data are available for the use of VALTREX in pediatric patients with creatinine clearance less than 50 mL/min/1.73 m2. Use in specific populations PHARMACOLOGYTable 1. VALTREX Dosage Recommendations for Adults with Kidney Disability Table 1. VALTREX Dosage Recommendations for Adults with Kidney DisabilityIndictions Normal dosage (Reduced from creatinine ≥50 mL/min)Reduced from creatinine (mL/min)30-4910-29 Cold Sores (Herpes Labialis) Do not exceed 1 day of treatment. Genital Herpes: Initial Episode Genital Brotherhood: Recurrent Episode Genital Herpes: Supressive TherapyHerpes zoster Patients who require should receive the recommended dose of VALTREX after hemodialysis. During hemodialysis, the average life of aciclovir after VALTREX administration is approximately 4 hours. About a third of acyclovir in the body is removed during a 4-hour hemodialysis session. There is no specific information for VALTREX administration in patients receiving . The effect of chronic outpatient dialysis () and continuous arteriovenous hemofiltration/dialysis (CAVHD) has been studied in acyclovir pharmacokinetics. The elimination of aciclovir after CAPD and CAVHD is less pronounced than with hemodialysis, and pharmacokinetic parameters closely resemble those observed in patients with () not receiving hemodialysis. Therefore, supplemental doses of VALTREX should not be necessary after CAPD or CAVHD. How SUPPLIEDDosage Forms and StrengthsStorage And HandlingVALTREX tablets (blue, filmed, capsule-shaped tablets printed with "VALTREX 500 mg") containing 556,2 mg of valaciclovir hydrochloride equivalent to 500 mg of valaciclovir. VALTREXBotella de 30 (NDC 0173-0933-08).Botella de 90 (NDC 0173-0933-10). 100 unit dose package (NDC 0173-0933-56). NDCNDCNDCVALTREX tablets (blue, film-coated, capsule-shaped tablets, with a partial score bar on both sides, printed with "VALTREX 1 gram") containing 1,112 grams of chlorhydrate valacyclovir equivalent to 1 gram of valaciclovir. VALTREXBotella de 30 (NDC 0173-0565-04).Botella de 90 (NDC 0173-0565-10). NDCNDCStore at 15° to 25°C (59° to 77°F). Dispensing in a well-closed container as defined in the USP. Distributed by: GlaxoSmithKline Research Triangle Park, NC 27709. Reviewed: Dec 2019SIDE EFFECTS The following severe adverse reactions are discussed in more detail in other sections of the label: ADMINISTRATIVES AND PRECAUTIONS The most common adverse reactions reported in at least 1 indication for more than 10% of the adult subjects treated with VALTREX and most frequently observed with VALTREX compared to placebo are headache, nausea and abdominal pain. The only adverse reaction reported in more than 10% of pediatric subjects under 18 years of age was headache. Clinical Trial Experience in Adult Subjects Because clinical trials are performed under very variable conditions, adverse reaction rates observed in clinical trials of a drug cannot be compared directly to clinical trials of another drug and cannot reflect the rates observed in practice. In clinical trials for the treatment of cold sores, adverse reactions reported by subjects receiving VALTREX 2 grams twice a day (n = 609) or placebo (n = 609) for 1 day, respectively, include headache (14%, 10%) and dizziness (2%, 1%). Abnormal ALT frequencies (more than 2 x ULN) were 1.8% for subjects who received VALTREX compared to 0.8% for placebo. Other laboratory abnormalities (white blood cells, alkaline phosphatase and serum creatinine) occurred with similar frequencies in the 2 groups. Initial Episode In a clinical trial for the treatment of initial herpes episodes, adverse reactions reported by more or equal to 5% of the subjects receiving VALTREX 1 gram twice a day for 10 days (n = 318) or oral acyclovir 200 mg 5 times a day for 10 days (n = 318), respectively, includes headache (13%, 10%) and nausea (6%, 6%). For the incidence of laboratory abnormalities see Table 2. Recurrent Episodes In 3 clinical trials for the episodic treatment of genital herpes, adverse reactions reported by more or equal to 5% of subjects receiving VALTREX 500 mg twice a day for 3 days (n = 402), VALTREX 500 mg twice a day for 5 days (n = 1,136), or placebo (n = 259), respectively, includes headache (16%, 11%, 14%). For the incidence of laboratory abnormalities see Table 2.Repression of Recidive Genital Herpes in Adults Immunocompetent In a clinical trial for the suppression of recurrent genital herpes infections, adverse reactions reported by subjects receiving VALTREX 1 gram once a day (n = 269), VALTREX 500 mg once a day (n = 266), or placebo (n = 134), respectively, includes headache (35%, 38%, 34%), nausea (11%, 8%), abdominal pain (11%, 9% For the incidence of laboratory anomalies see Table 2.Repression of Reciding Genetic Herpes in Infectious Subjects In HIV-1-infected individuals, adverse reactions for VALTREX (500 mg twice a day; n = 194, median days in therapy = 172) and placebo (n = 99, average days in therapy = 59), respectively, included headache (13%, 8%), fatigue (8%, 5%) and rash (8%, 1%). Post-lab abnormalities most frequently reported in valaciclovir versus placebo subjects included high alkaline phosphatase (4%, 2%), high ALT (14%, 10%), high AST (16%, 11%), decreased counts (18%, 10%), and decreased platelet counts (3%, 0%), respectively. Transmission reduction In a clinical trial for the reduction of the transmission of genital herpes, adverse reactions reported by subjects receiving VALTREX 500 mg once a day (n = 743) or placebo once a day (n = 741), respectively, included headache (29%, 26%), nasopharyngitis (16%, 15%) and upper respiratory tract infection (9%, 10%). In 2 clinical trials for the treatment of herpes zoster, adverse reactions reported by subjects receiving VALTREX 1 gram 3 times a day for 7 to 14 days (n = 967) or placebo (n = 195), respectively, include nausea (15%, 8%), headache (14%, 12%), vomiting (6%, 3%), dizziness (3%, 2%) and abdominal pain (3%). For the incidence of laboratory abnormalities see Table 2. Table 2. Incidence (%) of laboratory abnormalities in herpes zoster populations and genital herpes Table 2. %% %% %% %% %% %% %%% %% %%%% %%% %%% %%% %% %% %% %% %% %% %% %% %% %% %% %% %% %% %% %% %% %% %% %% %% %% %% %% %% %% %% %% %% %% %% %% %% %% %% %% %% %% %%% %% %% %%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%% %%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%% Data were not collected prospectively. LLN = lower limit than normal. ULN = upper limit of normal. Clinical Trial Experience in Pediatric Subjects VALTREX's safety profile has been studied in 177 pediatric subjects from 1 month to less than 18 years. Sixty-five of these pediatric subjects, aged 12 to under 18, received oral tablets for 1 to 2 days for the treatment of cold sores. The remaining 112 pediatric subjects, from 1 month to less than 12 years, participated in 3 pharmacokinetic and safety tests and received oral suspension valaciclovir. Fifty-one of these 112 pediatric subjects received oral suspension for 3 to 6 days. The frequency, intensity and nature of adverse clinical reactions and laboratory abnormalities were similar to those observed in adults. In clinical trials for the treatment of cold sores, adverse reactions reported by teen subjects receiving VALTREX 2 grams twice a day for 1 day, or VALTREX 2 grams twice a day for 1 day followed by 1 gram twice a day (n = 65, in both dosing groups), or placebo (n = 30), respectively, includes headache (17%, 3%) and nausea. The adverse events reported in more than 1 topic in 3 pharmacokinetic and safety trials in children from 1 month to less than 12 years were diarrhea (5%), pirexia (4%), dehydration (2%), simple herpes (2%), and rhinorrhea (2%). No clinically significant changes were observed in laboratory values. Postmarketing Experience In addition to adverse events reported in clinical trials, the following events have been identified during the postmarketing use of VALTREX. Because they are voluntarily reported from an unknown population, there is no frequency estimates. These events have been chosen for inclusion due to a combination of their seriousness, reporting frequency or possible causal connection with VALTREX. Facial eema, hypertension, tachycardia. Acute hypersensitivity reactions including anaphylaxis, angioedema, dyspnea, pruritus, eruption and hives [see CONTRAINDICATIONS]. CONTRAINDICATIONSAggressive behavior; agitation; ataxia; comma; confusion; decreased consciousness; dysarthria; encephalopathy; mania; and psychosis, including hearing and visual hallucinations, convulsions, tremors [see ATTENTION AND PRECAUTIONS, Use in Specific Populations]. ADMINISTRATIVES AND PRECAUTIONS Use in specific populations Visual abnormalities. Diarrhea. abnormalities of the liver enzyme, hepatitis. Kidney failure, kidney pain (can be associated with kidney failure) [see ATTENTION AND PRECAUTIONS, Use in Specific Populations]. ADMINISTRATIVES AND PRECAUTIONS Use in Specific PopulationsThrombocytopenia, aplastic anemia, leucocytoclastic vasculitis, TTP/HUS [see ATTENTION AND PRECAUTIONS]. ADMINISTRATIVES AND PRECAUTIONS Erythema multiforme, eruptions including photosensibility, alopecia. DROGA INTERACTIONS No clinically significant drug or drug interactions are known with VALTREX [see CLINICA PHARMACOLOGY]. PHARMACOLOGYIMAGESWARNINGSIncluded as part of the "PRECAUTIONS"PRECAUTIONS"PRECAUTIONSThrombotic Thrombocytopenic Purpura/Hemolytic Uremic Syndrome (TTP/HUS)TTP/HUS, in some cases that resulted in death, has occurred in patients with advanced HIVVAL-1 bone disease and also in bone marrow transplants Treatment with VALTREX should be stopped immediately if clinical signs, symptoms, and laboratory abnormalities are produced compatible with TTP/HUS. Acute renal failure Cases of acute kidney failure have been reported in:DOSAGE and ADMINISTRATION Use in specific populations DOSAGE AND ADMINISTRATION Use in specific populations In case of acute renal failure and anuria, the patient can benefit from hemodialysis until the renal function is restored [see DOSAGE AND ADMINISTRATION, ADVERSE REACTIONS]. DOSAGE AND ADMINISTRATIONADVERSE REACTIONS Central Effects of the Central Nervous System The adverse reactions of the central nervous system, including agitation, hallucinations, confusion, delirium, seizures and encephalopathy, have been reported in both adult and pediatric patients with or without reduced kidney function and in patients with underlying kidney disease who received doses higher than recommended VALTREX for their renal function level. Older patients are more likely to have adverse reactions from the central nervous system. VALTREX should be suspended if adverse reactions of the central nervous system occur [see REACTIONS WARNING, Use in specific populations]. REACTIONS ADVERSEUSE In Specific Populations Patient guidance information Advise the patient to read the FDA-approved patient labeling (PATIENT INFORMATION). PATIENT INFORMATION Patients should be advised to maintain proper hydration. Instruct patients who if they lose a dose of VALTREX, take it as soon as they remember. Advise patients not to duplicate their next dose or take more of the prescribed dose. Patients should be advised to begin treatment as soon as possible (e.g. tingling, itching or burning). There is no data on the effectiveness of the treatment initiated after the development of clinical signs of a cold ulcer (e.g. papula, vesicle or ulcer). Patients should be instructed that the treatment of cold sores should not exceed 1 day (2 doses) and that their doses should be taken approximately 12 hours apart. Patients should be informed that VALTREX is not a cure for cold sores. Patients should be informed that VALTREX is not a cure for genital herpes. Because genital herpes is a sexually transmitted disease, patients should avoid contact with sexual injuries or relationships when injuries and/or symptoms occur to prevent couples from infection. Genital herpes are often transmitted in the absence of symptoms through asymptomatic viral placement. Therefore, patients should be advised to use safer sexual practices in combination with VALTREX suppressive therapy. Sexual partners of infected people should be informed that they might be infected even if they do not have symptoms. Specific serological tests of asymptomatic partners of individuals with genital herpes can determine whether there is a risk of HSV-2 acquisition. VALTREX has not proven to reduce the transmission of sexually transmitted infections other than HSV-2. If the relapse of the medical administration of a genital herpes is indicated, patients are advised to begin therapy in the first sign or symptom of an episode. There is no data on the effectiveness of the treatment started more than 72 hours after the start of signs and symptoms of a first episode of genital herpes or more than 24 hours after the start of signs and symptoms of a recurring episode. There is no data on the safety or effectiveness of chronic suppressive therapy for more than 1 year in healthy other patients. There is no data on the safety or effectiveness of chronic suppressive therapy for more than 6 months in patients infected with HIV-1. There is no data on the treatment started more than 72 hours after the outbreak of the zoster eruption. Patients should be advised to start treatment as soon as possible after a diagnosis of herpes zoster. Patients should be advised to begin treatment with the earlier sign or symptoms of chickenpox. Nonclinical Toxicology Carcinogenesis, Mutagenesis, Fertility Impermeability The data below include references to stable state acyclovir observed in humans treated with 1 gram of VALTREX given orally 3 times a day to treat herpes zoster. Concentrations of plasma drugs in animal studies are expressed as multiple human exposure to acyclovir [see PHARMACOLOGY CLINICAL]. PHARMACOLOGY CLINICALValacyclovir was not carcinogenic in life-long carcinogenic trials in single daily doses (gavage) of valaciclovir giving plasma concentrations aciclovir equivalent to human levels in mouse bioassay and 1.4 to 2.3 times human levels in rat bioassay. There was no significant difference in the incidence of tumors between treated and control animals, nor was valacyclovir shortening tumor latency. Valacyclovir was tested in 5 genetic toxicity tests. An Ames test was negative in the absence or presence of metabolic activation. Also negative were an in vitro cytogenetic study with human lymphocytes and a cytogenetic study of rats. In the trial of mouse lymphoma, valaciclovir was not mutagenic in the absence of metabolic activation. In the presence of metabolic activation (76% to 88% conversion to aciclovir), valaciclovir was mutagenic. Valacyclovir was mutagenic in a micronucleus test of the mouse. Valacyclovir did not affect fertility or reproduction in male or female rats at acyclovir (AUC) exhibitions 6 times higher than in humans given the MRHD. The testicular atrophy occurred in male rats (orally dosed for 97 days at 18 times the MRHD) and was reversible. Use in specific populations Pregnancy Clinical data for several decades with valacyclovir and its metabolite, acyclovir, in pregnant women, have not identified a drug-related risk of major birth defects. There is insufficient data on the use of valaciclovir in relation to abortion or maternal and child outcomes or adverse fetals (see data). There are risks to the fetus associated with simple herpes during pregnancy (see Clinical Considerations). DataClinical Considerations In animal reproduction studies, there was no evidence of adverse development results with valaciclovir when it is given to pregnant rats and rabbits to system exposures (AUC) 4 (rats) and 7 (rabbits) times human exposure to the highest recommended human dose (MRHD) (see Data). Data The estimated background risk of major birth defects and miscarriage of the populations indicated is unknown. All pregnancies have a risk of birth defect history, loss or other adverse results. In the U.S. general population, the estimated background risk of major birth defects and abortions in clinical pregnancies is 2 per cent to 4 per cent and 15 per cent to 20 per cent, respectively. Maternal risk associated with disease and/or Embryo/Fetal The risk of HSV neonatal infection ranges from 30% to 50% for genital HV acquired in late pregnancy (third quarter), while with the acquisition of HVV in early pregnancy, the risk of neonatal infection is approximately 1%. An occurrence of primary herpes during the first trimester of pregnancy has been associated with neonatal chorerioretinitis, microcephaly and, in rare cases, skin lesions. In very rare cases, transplacental transmission can cause congenital infection, including microcephaly, hepatosplenomegaly, intrauterine growth restriction and mortanity. HSV infection increases the risk of perinatal HIV transmission in women who had a clinical diagnosis of genital herpes during pregnancy. Human Data Clinical data over several decades with valacyclovir and its metabolite, acyclovir, in pregnant women, based on published literature, have not identified a drug-related risk of major birth defects. There is insufficient data on the use of valaciclovir in relation to abortion or maternal and child outcomes or adverse fetals. The Acyclovir and the Valacyclovir Pregnancy Registries, both international population-based prospective studies, collected pregnancy data until April 1999. The Acyclovir Registry documented the results of 1,246 infants and fetuses exposed to aciclovir during pregnancy (756 with early exposure during the first quarter, 197 during the second quarter, 291 during the third quarter and 2 unknowns). The occurrence of significant birth defects during the first-quarter exposure to acyclovir was 3.2% (IC95%: 2.0% to 5.0%) and during any trimester of exposure was 2.6% (CI 95%: 1.8% to 3.8%). The Valacyclovir Pregnancy Registry documented the results of 111 babies and fetuses exposed to valaciclovir during pregnancy (28 with early exposure in the first quarter, 31 during the second quarter, and 52 during the third quarter). The incidence of the main birth defects during the first-quarter exposure to valaciclovir was 4.5% (IC95%: 0.24% to 24.9%) and during any quarter of exposure was 3.9% (IC95%: 1.3% to 10.7%). Available studies have methodological limitations that include insufficient sample size to support conclusions about the overall risk of malformation or to make comparisons of specific birth defect frequencies. Animal dataThe Valacyclovir was orally administered to pregnant rats and rabbits (up to 400 mg/kg/day) during organogenesis (Días de la Fiesta 6-15, and 6-18, respectively). In rats and rabbits, no adverse effects were observed in the fetal of the embryo in the acyclovir (AUC) exhibitions of up to 4 (rats) and 7 (rabbits) times human exposure in MRHD. The early death of the embryo, the retardation of fetal growth (weight and length) and variations in fetal skeletal development (mainly extra ribs and delayed osification of sternebrae) were observed in rats and associated with maternal toxicity (200 mg/kg/day; approximately 6 times higher than human exposure in MRHD). In a prenatal and postnatal development study, valaciclovir was orally administered to pregnant rats (up to 200 mg/kg/day of Gestation Day 15 to Post-Partum 20) of late pregnancy through breastfeeding. No significant adverse effects were observed on the daily reported offspring from before birth through breastfeeding to maternal exposures (AUC) of approximately 6 times higher than human exposures in MRHD. Lactation Although there is no information about the presence of valaciclovir in human milk, its metabolite, aciclovir, is present in human milk after the oral administration of valaciclovir. According to published data, a maternal dose of VALTREX of 500 mg twice a day would provide a child breastfeeding an oral acyclovir dose of approximately 0.6 mg/kg/day (see data). There is no data on the effects of valaciclovir or aciclovir on the breastfed child or on milk production. Benefits for the development and health of breastfeeding should be considered together with the mother's clincial need for VALTREX and any possible negative effect on the breastfeeding child of VALTREX or the underlying maternal condition. Data Following the oral administration of a dose of 500 mg of VALTREX to 5 lactating women, the maximum concentrations of aciclovir (Cmax) in breast milk ranged from 0.5 to 2.3 times (median 1,4) the corresponding concentrations of maternal serum aciclovir. Maternal milk aciclovir AUC ranges from 1.4 to 2.6 times (average 2.2) of AUC maternal serum. A maternal dose of VALTREX of 500 mg twice a day would provide a breastfeeding child with an oral dose of acyclovir of about 0.6 mg/kg/day. Valaciclovir was not detected without changes in maternal serum, breast milk or infant urine. Pediatric UseVALTREX is indicated for the treatment of cold sores in pediatric patients older than or equal to 12 years and for the treatment of chickenpox in pediatric patients aged 2 to under 18 [see INDICATIONS, DOSAGE and ADMINISTRATION]. INDICATIONSDOSAGE AND ADMINISTRATION The use of VALTREX for the treatment of cold sores is based on 2 double-blind, placebo-controlled clinical trials in healthy adults and adolescents (older or equal to 12 years) with a history of recurrent cold sores [see Clinical Studies]. Clinical studies The use of VALTREX for the treatment of chickenpox in pediatric patients from 2 to less than 18 years is based on pharmacokinetic and multi-dose safety data from an open label trial with valaciclovir and supported by data of efficacy and safety of 3 randomized, double-blind placebo-controlled trials that evaluate oral acyclovirics No effectiveness evaluations were conducted in any of the 3 trials. Trial 1 was a pharmacokinetic and multi-dose safety test in 27 pediatric subjects from 1 to less than 12 years with the infection of the varicela-zoster virus (VZV) clinically suspicious [see DOSAGE and ADMINISTRATION, REACTIONS WARNING, PHARMACOLOGY CLINICAL, Clinical Studies]. DOS and REACTIONSCLINICA PHARMACOLOGYClinical StudiesTrial 2 was a pharmacokinetic and safe trial of unique doses in pediatric subjects of 1 month to less than 6 years who had an active infection of the herpes virus or who were at risk of infection by the herpes virus. Fifty subjects were registered and received a single dose of 25 mg/kg oral suspension valaciclovir. In infants and children from 3 months to less than 6 years old, this dose provided systemic acyclovir exposures comparable to that of a dose of 1 grams of valaciclovir in adults (historic data). In 1 month to less than 3 months babies, aciclovir exposures for a dose of 25 mg/kg were higher (Cmax: ↑30%, AUC: ↑60%) than aciclovir exposures after a dose of 1 grams of valaciclovir in adults. Acyclovir is not approved for suppressive therapy in infants and children after neonatal VH infections; therefore, valaciclovir is not recommended for this indication because efficacy cannot be extrapolated from acyclovir. Trial 3 was a multi-dose pharmacokinetic safety test in 28 pediatric subjects from 1 to less than 12 years with clinical suspicion of VH infection. None of the subjects registered in this trial had genital herpes. Each subject was dosed with oral suspension valaciclovir 10 mg/kg twice a day for 3 to 5 days. Systemic aciclovir exposures in pediatric subjects after oral suspension valaciclovir were compared to historical acyclovir systemic exposures in immunocompetent adults who received the solid form of oral dosing of valacyclovir or acyclovir for the treatment of recurrent genital herpes. The projected average systemic exposure acyclovir daily in pediatric subjects in all age groups (1 to less than 12 years) was lower (Cmax: ↓20%, AUC: ↓33%) compared to the systemic exposures aciclovir in adults who receive valaciclovir 500 mg twice a day, but were older (AUC day: ↑16%) than the 200 mg shows. Insufficient data are available to support valaciclovir for the treatment of recurrent genital herpes in this age group, as clinical information on recurrent genital herpes in young children is limited; therefore, it is not possible to extrapolate data of adults effectiveness to this population. In addition, valaciclovir has not been studied in children aged 1 to under 12 years with recurrent genital herpes. Geriat UsoFrom the total number of subjects in clinical trials of VALTREX, 906 were 65 and more, and 352 were 75 and more. In a clinical trial of herpes zoster, the duration of pain after healing (positive necuralgia) was greater in subjects 65 and older than younger adults. Older patients are more likely to have a reduced kidney function and require dose reduction. Older patients are also more likely to have kidney or CNS adverse events [see DOSAGE and ADMINISTRATION, ADMINISTRATIONS and PRECAUTIONS, PHARMACOLOGY CLINICA]. ADMINISTRATION AND ADMINISTRATION PHARMACOLOGYRenal Impairment It is recommended to reduce the dose by administering VALTREX to patients with kidney deficiency [see DOSAGE and ADMINISTRATION, ADMINISTRATIONS and PRECAUTIONS]. DOSGINA AND ADMINISTRATION OF ARMS AND PRECAUTIONS Supervision should be exercised to prevent unnoticed overdose [see Use in Specific Populations]. Acyclovir precipitation in renal tubules can occur when solubility (2.5 mg/ml) is exceeded in intratubular fluid. In case of acute renal insufficiency and anuria, the patient may benefit from hemodialysis until the renal function [see DOSAGE and ADMINISTRATION] is restored. Use In Specific PopulationsDOSAGE AND ADMINISTRATIONCONTRAINDICATIONSVALTREX is contraindicated in patients who have had a clinically significant hypersensitivity reaction (e.g. anaphylaxis) to valaciclovir, acyclovir or any component of the formulation [see REACTIONS WARNING]. ADVERSE REACTIONSCLINICAL PHARMACOLOGYMechanism Of ActionValacyclovir is an active antiviral drug against α-herpes virus [see Microbiology]. MicrobiologyPharmacokinetics Valaciclovir and aciclovir pharmacokinetics after VALTREX oral administration have been investigated in 14 voluntary trials with 283 adults and in 3 trials with 112 pediatric subjects from 1 month to less than 12 years. Absorption and bioavailability After oral administration, chlorhydrate valaciclovir is quickly absorbed from the gastrointestinal tract and almost completely converted into acyclovir and L-valine by first-step intestinal and/or liver metabolism. The absolute bioavailability of aciclovir after VALTREX administration is 54.5% ± 9.1% as determined after an oral dose of VALTREX of 1 grams and an intravenous dose of aciclovir of 350 mg to 12 healthy volunteers. VALTREX administration's acyclovir bioavailability is not altered by food management (30 minutes after a breakfast of 873 Kcal, which included 51 grams of fat). The estimates of the pharmacokinetic parameter Acyclovir after VALTREX administration to healthy adult volunteers are presented in table 3. There was a lesser increase than the dose-proportional in the maximum concentration of aciclovir (Cmax) and area under the aciclovir concentration time curve (AUC) after the administration of single doses and multiple doses (4 times a day) of VALTREX doses between 250 mg to 1 gram. There is no accumulation of acyclovir after the administration of valaciclovir in recommended dose regimes in adults with normal kidney function. Table 3. Media (±SD) Plasma Acyclovir Pharmacokinetic parameters after the administration of VALTREX to Healthy Adult Volunteers 3 ± 6 ± 3,15 ± 0,40 ± 0,15 ± 0,40 ± 0,40 ± 0,40 ± 0,40 ± 0,40 ± 0,40 ± 0,40 ± 0,40 ± 0,40 ± Distribution The union of valaciclovir to human plasma proteins ranges from 13.5 to 17.9%. The binding of acyclovir to human plasma proteins ranges from 9% to 33%. MetabolismValacyclovir becomes acyclovir and L-valine for the first time intestinal and/or liver. Acyclovir becomes a small measure inactive metabolites by oxidized aldehyde and alcohol and dehydrogenous aldehyde. Neither valacyclovir nor acyclovir metaboly with cytochrome enzymes P450. Unreverted valacyclovir plasma concentrations are low and transient, usually not quantifiable in 3 hours after administration. Valaciclovir concentrations in peak plasma are usually less than 0.5 mcg/m L at all doses. After the administration of single doses of 1 gram of VALTREX, the average concentrations of valaciclovir plasma observed were 0,5, 0,4 and 0,8 mcg/mL in subjects with liver dysfunction, kidney failure and in healthy subjects who received concomitant cymetidine and probenecid respectively. Removal The pharmacokinetic disposition of acyclovir delivered by valacyclovir is consistent with the previous experience of intravenous and oral acyclovir. After the oral administration of a single dose of valaciclovir from 1 grams to 4 healthy subjects, 46% and 47% of managed radioactivity were recovered in urine and feces, respectively, over 96 hours. The Acyclovir represented 89% of the radioactivity excreted in the urine. The renal cleaning of acyclovir after the administration of a dose of 1-gram of VALTREX to 12 healthy subjects was approximately 255 ± 86 mL/min which represents 42% of the total cleaning of apparent plasma aciclovir. Plasmatic elimination of acyclovir usually averaged 2.5 to 3.3 hours in all VALTREX tests in subjects with normal kidney function. Specific populationsDrug reduction is recommended in patients with kidney deficiency [see DOSAGE and ADMINISTRATION, Use in Specific Populations]. DOSAGE AND ADMINISTRATION Use in specific populations After the administration of VALTREX to subjects with ESRD, the average aciclovir life is approximately 14 hours. During hemodialysis, the average acyclovir life is about 4 hours. Approximately one third of the acyclovir in the body is eliminated by dialysis during a 4-hour hemodialysis session. The apparent plasma cleaning of aciclovir in dialysis subjects was 86.3 ± 21.3 mL/min/1.73 m2 compared to 679.16 ± 162.76 mL/min/1.73 m2 in healthy subjects. The administration of VALTREX to subjects with moderate cirrhosis (provided by biopsy) or severe (with and without biopsy) indicated that the rate but not the conversion extension of valaciclovir to acyclovir is reduced, and the acyclovir half life is not affected. No dose modification is recommended for patients with cirrhosis. In 9 subjects with HIV-1 disease and CD4+ cells count less than 150 cells/mm3 that received VALTREX at a dose of 1 gram 4 times a day for 30 days, valaciclovir and acyclovir pharmacokinetics were not different from those observed in healthy subjects. After the administration of a single dose of 1 gram of VALTREX in healthy geriatric subjects, the average life of aciclovir was 3.11 ± 0.51 hours compared to 2.91 ± 0.63 hours in healthy young adults. Acyclovir pharmacokinetics after the oral administration of single and multiple doses of VALTREX in geriatric subjects varied with kidney function. The reduction of the dose may be necessary in geriatric patients, depending on the underlying kidney condition of the patient [see DOSAGE AND ADMINISTRATION, Use In Specific Populations]. DOSAGE AND ADMINISTRATION Use in specific populations Acyclovir pharmacokinetics have been evaluated in a total of 98 pediatric subjects (from 1 month to less than 12 years) following the administration of the first dose of a temporary oral suspension of valaciclovir [see REACTIONS ADVERSE, Use In Specific Populations]. The estimates of the pharmacokinetic parameter Acyclovir after a dose of 20 mg/kg are shown in table 4. REACTIONS ADVERSEUSE In Specific Populations Table 4. Meaning (± SD) Plasma Acyclovir Farmcokinetic Parameter First-dose administration estimates of 20 mg/kg Valacyclovir Oral suspension to pediatric subjects vs. 1-Gram A unique dose of VALTREX to adults Table 4. Meaning (± SD) Plasma Acyclovir Farmcokinetic Parameter First-dose administration estimates of 20 mg/kg Valacyclovir Oral suspension to pediatric subjects vs. 1-Gram Unique dose of VALTREX to adultsParameterPydiatric symptoms (20 mg/kg Oral suspension)Adults 1-gram Solid dose of VALTREXa(n = 15)1 - Drug Interaction Studies When VALTREX is co-administered with antiacids, cymetidine and/or pro-enicid, digoxin or diuretic tizazide in patients with normal kidney function, the effects are not considered of clinical importance (see below). Therefore, when VALTREX is administered jointly with these drugs in patients with normal kidney function, no dose adjustment is recommended. Acyclovir pharmacokinetics after a single dose of VALTREX (1 gram) was not changed by the administration of a single dose of antiacids (Al3+ or Mg++++). Acyclovir Cmax and AUC after a single dose of VALTREX (1 gram) increased by 8% and 32%, respectively, after a single dose of cimetidine (800 mg). Acyclovir Cmax and AUC after a single dose of VALTREX (1 gram) increased by 30% and 78%, respectively, after a combination of cimetidine and probenecid, mainly due to the reduction of renal cleansing of aciclovir. The pharmacokinetics of digoxin was not affected by VALTREX co-administration 1 gram 3 times a day, and the pharmacokinetics of acyclovir after a single dose of VALTREX (1 gram) was not changed by the co-administration of digoxin (2 dose of 0.75 mg). Acyclovir Cmax and AUC after a single dose of VALTREX (1 gram) increased by 22% and 49%, respectively, after probenecid (1 gram). Acyclovir pharmacokinetics after a single dose of VALTREX (1 gram) was not changed by the administration of multiple doses of chalk diuretics. MicrobiologyValacyclovir is a desoxynucleoside analog DNA polymerase inhibitor. Valacyclovir hydrochloride quickly becomes acyclovir, which has shown antiviral activity against the types of VHS 1 (HSV-1) and 2 (HSV-2) and VZV both in cell and in vivo culture. Acyclovir is a synthetic deoxynucleoside that is intracellularly phosphorylated by the viral encoded thymidine kinase (TK; pUL23) of HSV or VZV in acyclovir monophosphate, a nucleotide analogue. Monophosphate becomes diffosphate by cell guanylate cinosa and in triphosphate by a series of cellular enzymes. In biochemical trials, acyclovir triphosphate inhibits the replication of the viral DNA of α-herpes. This is achieved in three ways: 1) competitive inhibition of viral DNA polymerase, 2) incorporation and termination of the growing viral DNA chain, and 3) inactivation of viral DNA polymerase. The greatest antiviral activity of aciclovir against HSV compared to VZV is due to its most efficient phosphorylation by viral TK. The quantitative relationship between the susceptibility of the herpesvirus cell culture to the antivirals and the clinical response to therapy has not been established in humans, and the sensitivity tests of the virus have not been standardized. The results of the sensitivity tests, expressed as the concentration of drugs necessary to inhibit the growth of the virus in cell culture by 50% (EC50), vary greatly depending on several factors. Using plaque reduction tests, EC50 values against single herpes virus insulated range from 0.09 to 60 microM (0.02 to 13.5 mcg/mL) for HSV-1 and from 0.04 to 44 microM (0.01 to 9.9 mcg/mL) for HSV-2. The EC50 values for aciclovir against most VZV lab strains and clinical insulations range from 0.53 to 48 microM (0.12 to 10.8 mcg/mL). Acyclovir also demonstrates activity against the VZV Oka vaccine strain with an average EC50 value of 6 microM (1.35 mcg/mL). In Cell CultureHSV-1 strains resistant to cyclovir, HSV-2 and VZV were isolated in cell culture. HSV and VZV resistant to acyclovir were derived from mutations in the genes of viral thymidine (TK, pUL23) and DNA polymerase (POL; pUL30). The fragments were commonly isolated and result in premature truncation of the HSV TK product, resulting in a decrease in susceptibility to acyclovir. Mutations in the viral TK gene can lead to a total loss of TK activity (TK ivels), reduced levels of TK activity (partial tK), or alteration in the ability of viral TK to phosphorylate the drug without an equivalent loss in the capacity of phosphorylating the timidine ( altered TK). Patients infected by HSV The HSV-1 and HSV-2 insulated were evaluated from patients who did not receive treatment for their α-herpes virus infections for genotypical changes in TK and POL genes and for phenotypic resistance to acyclovir. HSV insulations were identified with mutations in the frame and substitutions associated with resistance in TK and POL. The possibility of viral resistance to acyclovir should be considered in patients who do not respond or experience recurrent viral spilling during therapy. Cross resistance has been observed among HSV isolates that carry mutations in the frame and substitutions associated with resistance, which confer less susceptibility to penciclovir, famciclovir and foscarnet. Clinical studies Cold Sores (Herpes Labialis) Two double-blind, placebo-controlled clinical trials were conducted in 1,856 healthy adults and adolescents (older or equal to 12 years) with a history of recurrent cold sores. Subjects self-initiated therapy to the first symptoms and before any sign of cold pain. Most subjects started treatment within 2 hours of symptoms. The topics were randomized to VALTREX 2 grams twice a day 1 followed by placebo on the day 2, VALTREX 2 grams twice a day 1 followed by 1 grams twice a day 2 or placebo on the days 1 and 2. The average duration of episodes of cold pain was approximately 1 shorter day in treated subjects compared to placebo. The 2-day regime did not offer any additional benefits during the 1-day regime. No significant difference was observed between subjects who received VALTREX or placebo in preventing the progression of cold pain injuries beyond the papular stage. Genital Herpes Infections Six hundred forty-three immunocompetent adults with first-stage genital herpes who presented within 72 hours of symptoms began were randomized in a double-blind trial to receive 10 days of VALTREX 1 gram twice a day (n = 323) or oral acyclovir 200 mg 5 times a day (n = 320). For both treatment groups the average time for wound healing was 9 days, the average time for the cessation of pain was 5 days, and the average time for the cessation of viral spilling was 3 days. Three double-blind trials (2 of them controlled by placebo) were performed in immunocompetent adults with recurrent genital herpes. Subjects self-initiated therapy within 24 hours of the first sign or symptom of an episode of recurrent genital herpes. In 1 trial, subjects were randomized to receive 5 days of treatment with VALTREX 500 mg twice a day (n = 360) or placebo (n = 259). The average time for wound healing was 4 days in the group that received VALTREX 500 mg compared to 6 days in the placebo group, and the average time for the cessation of viral coverage in subjects with at least 1 positive culture (42% of the total population of rehearsal) was 2 days in the group that received VALTREX 500 mg compared to 4 days in the placebo group. The average time to stop the pain was 3 days in the group that received VALTREX 500 mg against 4 days in the placebo group. The results that support effectiveness were replicated in a second trial. In a third trial, subjects were randomized to receive VALTREX 500 mg twice a day for 5 days (n = 398) or VALTREX 500 mg twice a day for 3 days (and placebo twice a day for 2 additional days) (n = 402). The average time for wound healing was approximately 41⁄2 days in both treatment groups. The average time to stop pain was about 3 days in both treatment groups. Two clinical trials were conducted, one in immunocompetent adults and one in HIV-infected adults. A 12-month, double-blind, placebo, and asset-controlled trial inscribed immunocompetent adults with a history of 6 or more recurrences a year. The results of the total trial population are shown in table 5. Table 5. Repetition rates of immunocompetent adults at 6 and 12 months Table 5. Repetition rates in adults immunocompetent at 6 and 12 months Months12 MonthsVALTREX 1 gram Once Daily(n = 269)Oral acyclovir 400 mg Twice Daily(n = 267)Placebo(n = 134)VALTREX 1 gram Once Daily(n = 269)Oral ecmail 400 mg Twice Daily(n = 267 % Subjects with 9 or less recurrences a year showed comparable results with VALTREX 500 mg once a day. In a second trial, 293 HIV-1-infected adults in stable antiretroviral therapy with a 4- or more anogenic herpes-recurrents per year were randomized to receive VALTREX 500 mg twice a day (n = 194) or placebo (n = 99) for 6 months. The average duration of recurrent genital herpes in registered subjects was 8 years, and the average recurrence in the year prior to registration was 5. Overall, the middle-aged HIV-1 RNA was 2,6 log10 copies/mL. Among the subjects who received VALTREX, the CD4+ cell count of preventive median was 336 cells/mm3; 11% had less than 100 cells/mm3, 16% had 100 to 199 cells/mm3, 42% had 200 to 499 cells/mm3, and 31% had more or equal to 500 cells/mm3. The results of the total trial population are shown in table 6. Table 6. Repetition rates for adults infected with HIV-1 at 6 months Table 6. Repeat rates in adults infected with HIV-1 to 6 monthsOutcomeVALTREX500 mg Twice Daily(n = 194)Placebo(n = 99)Free recurrence65%26%Recurrences17%57%Unknown18%17%a Includes missing cases of follow-up, interruptions due to adverse events and withdrawn consent. A double-blind, placebo-controlled trial was performed to evaluate the transmission of genital herpes in 1,484 monogamous, heterosexual, immunocompetent adult couples. Couples were discordant for HSV-2 infection. The source partner had a history of 9 or less episodes of genital herpes per year. Both partners were advised on safer sexual practices and were advised to use condoms throughout the trial period. The origin partners were randomized to treatment with VALTREX 500 mg once a day or placebo once a day for 8 months. The primary endpoint was the symptomatic acquisition of HSV-2 in susceptible partners. The overall acquisition of HSV-2 was defined as symptomatic acquisition of HSV-2 and/or HSV-2 seroconversion in susceptible partners. The effectiveness results are summarized in table 7. Table 7. Percentage of sustainable partners who learned HSV-2 defined by primary and selected endpoints 7. Percentage of Subceptible Partners Acquired HSV-2 Defined by Primary Secondary Points and Selected Endpoints EndpointVALTREX(n = 743)Placebo(n = 741)Systomatic acquisition HSV-24 (0.5%)16 (2.2%)Seroconversion12 (1.6%)24 (3.2%)Completion total HSV-214 (1.1%) (1.2%) Individual results can vary according to the coherence of safer sexual practices. Herpes Zoster Two randomized double-blind clinical trials were performed in adults with localized zoster herpes. VALTREX was compared with placebo in subjects under 50 years of age and with oral aciclovir in subjects over 50 years of age. All subjects were treated within 72 hours of zoster rash. In subjects under 50 years of age, the average time to stop the new training was 2 days for VALTREX treaties compared to 3 days for placebo treaties. In subjects over the age of 50, the average time of cessation of new injuries was 3 days in subjects treated with VALTREX or oral acyclovir. In subjects under 50 years of age, no difference was found regarding the duration of pain after healing (post-herpetic ) between VALTREX and placebo receptors. In subjects over 50 years of age, between 83% who reported pain after healing ( postherpetic neuralgia), the median duration of pain after healing (95% CI) in days was: 40 (31, 51), 43 (36, 55), and 59 (41, 77) by 7-day VALTREX, 14-day VALTREX and 7-day oral aciclovir, respectively. Chickenpox The use of VALTREX for the treatment of chickenpox in pediatric subjects from 2 to less than 18 years is based on pharmacokinetic and multi-dose safety data of a valaciclovir and supported by extrapolated safety data and efficacy of 3 randomized, double-blind, placebo-controlled trials, evaluating oral aciclovir in pediatric subjects. The pharmacokinetic and multi-dose safety test enrolled 27 pediatric subjects from 1 to less than 12 years with clinical suspicion of VZV infection. Each subject was dosed with oral suspension valaciclovir, 20 mg/kg 3 times a day for 5 days. Systemic aciclovir exposures in pediatric subjects after oral suspension valaciclovir were compared to historical acyclovir systemic exposures in immunocompetent adults who received the solid form of oral dosing of valacyclovir or acyclovir for the treatment of herpes zoster. The average projected daily aciclovir exposures in pediatric subjects in all age groups (1 to less than 12 years) was lower (Cmax: ↓13%, AUC: ↓30%) than the average of daily historical exhibitions in adults receiving valaciclovir 1 gram 3 times a day, but were greater (per day AUC: ↑50%) than the average daily historical exhibitions in adults. The projected daily exposures in pediatric subjects were greater (per day AUC approximately 100% greater) than the exposures observed in pediatric immunocompetent subjects who received acyclovir 20 mg/kg 4 times a day for the treatment of smallpox. Based on the pharmacokinetic and safety data of this trial and the extrapolated efficacy data of acyclovir, oral valaciclovir 20 mg/kg 3 times a day for 5 days (not exceeding 1 gram 3 times a day) is recommended for the treatment of smallpox in pediatric patients from 2 to less than 18 years. Because the effectiveness and safety of aciclovir for the treatment of chickenpox in children under 2 years of age have not been established, effective data cannot be extrapolated to support valaciclovir treatment in children under 2 years of age with chickenpox. Valacyclovir is not also recommended for the treatment of herpes zoster in children because no safety data is available for up to 7 days [see Use in Specific Populations]. Use in specific populations PATIENT INFORMATIONVALTREX® (VAL-Trex) (hydrochloride valaciclovir) CapletsVALTREX®Read the patient information that comes with VALTREX before you start using it and every time you get a recharge. There may be a new one. information. This information does not take the place to speak to your healthcare provider about your medical condition or treatment. Ask him. health care provider or pharmacist if you have questions. What's VALTREX? What's VALTREX? VALTREX is a prescription drug. VALTREX reduces the ability of herpes to multiply in your body. VALTREX is used in adults: Do not have sexual contact with your partner when you have any symptoms or outbreak of genital herpes. Use a condomVALTREX does not cure herpes infections (cold sores, chickenpox, herpes or genitalia. VALTREX does not cure herpes infections VALTREX effectiveness has not been studied in children They haven't reached puberty. What are the cold sores, chickenpox, wrinkles and genitals? Herpes? What are the cold sores, chickenpox, wrinkles and genitals? Herpes? Cold sores are caused by a herpes virus that can to be disseminated kissing or other physical contact with the infected area of the skin. They are small and painful ulcers that you get in your mouth or around it. It It is not known if VALTREX can stop the spread of cold sores to others. Chickenpox Cold Soras is caused by a herpes virus. Cause a spicy eruption of multiple small, red that look or insects The bites usually appear first in the abdomen or back and face. It can be extended almost everywhere in the body and may be accompanied by flu-like symptoms. Chickenpox Shingles is caused by the same herpes virus as cause chickenpox. It causes small and painful blisters that occur in the skin. Shredding occurs in people who have already had chickenpox. Sleeves can be dissemination to people who have not had chickenpox or chickenpox vaccine contact with infected areas of the skin. It is not known if VALTREX can Stop the spread of herpes to others. Genital Herpes is a . It causes small and painful blisters in your genital area. You can spread genitals herpes others, even when you have no symptoms. If you are sexually active, You can still pass herpes to your partner, even if you're taking VALTREX. VALTREX, taken every day as prescribed and used with the following safest sex practices, can reduce the chances of passing herpes genital to your partner. Genital Herpes Ask your health care provider for more information on Safer sexual practices. Who shouldn't take VALTREX? Who shouldn't take VALTREX? Do not take VALTREX if you are allergic to any of its ingredients or . The active ingredient is valacyclovir. See the end of this brochure for a complete list of ingredients in VALTREX. Do not take VALTREX Before taking VALTREX, tell your healthcare provider: Before taking VALTREX, tell your healthcare provider: About all your medical conditions, including: About all your medical conditions, including: if you have had a bone marrow transplant or kidney transplant, or if you have advanced HIV-1 or AIDS disease. if you have kidney problems. if you're 65 or older. if you are pregnant or plan to become pregnant. if you're breast-feeding about all the medications you take, how should I take VALTREX? How should I take VALTREX? Take VALTREX exactly as prescribed by your health care supplier. Your VALTREX dose and the duration of the treatment will depend on the type Herpes infection you have and any other medical problems you have. What are the possible side effects of VALTREX? What are the possible side effects of VALTREX? Kidney failure and nervous system problems are not common, but may be serious in some patients taking VALTREX. Nervous system problems include aggressive behavior, unstable movement, careless movements, confusion, speech problems, hallucinations (seeing or hearing things that don't really there), seizures and coma. Kidney failure and nervous system problems happened in patients who already have kidney disease and in elderly patients whose kidneys don't work well because of age. Always tell your health supplier if you have kidney problems before taking VALTREX. Call your doctor Immediately if you have a nervous system problem while you are taking VALTREX. Kidney failure and nervous system problems are not common, but may be serious in some patients taking VALTREX. Always tell your health supplier if you have kidney problems before taking VALTREX. Call your doctor Immediately if you have a nervous system problem while you are taking VALTREX. Common adult VALTREX side effects include headache, nausea, stomach pain, vomiting and dizziness. Side effects in Adults infected with HIV include headache, tiredness and rash. These side effects They are usually mild and do not cause patients to stop taking VALTREX. Other less common side effects in adults include pain periods in women, joint pain, depression, low blood cell counts and changes in tests that measure how well the liver and kidneys work. The most common side effect seen in young children 18 years old was headache. Talk to your healthcare provider if you develop any side effects that concern you. Talk to your healthcare provider if you develop any side effects that concern you. These are not all VALTREX side effects. For more Ask your healthcare provider or pharmacist. How can I store VALTREX? How can I store VALTREX? General information about VALTREX General information about VALTREXMedicines are sometimes prescribed for conditions that are not mentioned in patient information brochures. Do not use VALTREX for a condition for which it was not prescribed. Don't give VALTREX to other people, even if they have the same symptoms they have. It can damage them. This brochure summarizes the most important information about VALTREX. For more information, talk to your health supplier. You may ask your healthcare provider or pharmacist for information about VALTREX that is written for health professionals. More information is available at www. VALTREX.com. What are the ingredients in VALTREX? What are the ingredients in VALTREX? Active ingredient: valacyclovir hydrochloride Ingredient:Inactive Ingredients: carnauba wax, colloidal Silicon dioxide, crospovidone, FD Blue No. 2 Lake, hypromellose, magnesium stearate, microcrystalline cellulose, glycol polyethylene, polysorbate 80, povidone and titanium dioxide. Inactive Ingredients:From Report Problems to the Food and Drug AdministrationYou are encouraged to report the negative side effects of prescription drugs to the FDA. Visit the website or call 1-800-FDA-1088. Health Solutions of Our SponsorsQuick, Easy Identification Tool, Pill Identification Drug Interaction Tool Drug Powerful Interaction Control Pharmacy Locater Tool Including 24 Hours, Valtrex Pharmacies Execution: Execution: Execution: Execution, Execution, Execution, Execution, Execution, Execution, Execution, Execution, Execution, Execution, Execution, Execution, Execution, Execution, Execution, Execution, Execution, Execution, Execution, Execution, Execution, Execution, Execution, Execution, Execution, Execution, Execution, Execution, Execution, Execution, Execution, Execution, Execution, Execution, Execution, Execution, Execution, Execution, Execution, Execution, Execution, Execution, Execution, Execution, Execution, Execution, Execution, Execution, Execu RxList does not provide medical advice, diagnosis or treatment. .
The main objective of this study is to follow up on the changes in the behaviour and trends of travel in the National Covenant on Economic, Social and Cultural Rights recorded in four main surveys on travel of origin and desertification conducted in 1986, 1995, 2005, and more recently, in 2011. These surveys present a snapshot of travel demand on a typical NCR day by surveying a large sample of their residents about where, why, when and how they traveled. This, together with demographic information about travelers, portrays the changing state of travel in the NCR. This report updates and is based on previous studies by NCR Travel Trends presenting the relationship between travel trends and changing economic and demographic conditions. Updated 2011 Census and other sources help to establish a holistic picture of emerging, changing and established trends within the NCR. On the basis of the trends noted, the present report also presents a prospective analysis of possible changes in the behaviour of travel to be reported in the coming years. The full report () has been published. To complement the 2011 Origin-Destination survey (O-D), the TRANS Committee will conduct one in the National Capital Region from 4 to 29 November 2013. TRANS is learning more about travel patterns and the characteristics of visitors to the National Capital Region, as well as the traffic generated by the main destinations of the region for both permanent and non-permanent residents. For more detailed information, please review the . The results provide a detailed picture of the "who, why, when and how" of the trips made by residents of the National Capital Region. In total, 25,374 telephone interviews were completed in autumn 2011, representing 5.0% of all households, which is considered a rich sample. For more detailed information, see . The NCR 2009 External Travel Survey was an important follow-up to the household-based 2005 O-D Survey and captured information on trip patterns that were not captured in the 2005 survey, specifically travel that originate in, or are intended to, locations outside of the NCR. The survey was conducted in spring/summer 2009. In total, 17,744 valid surveys were completed, representing 13.3 per cent of all traffic passing through survey sites during the 11-hour survey period, which is considered a rich and complete sample. Read more about the results of . In the summer of 2007 (June), an Interprovincial Survey of Road Trucks was conducted to establish a comprehensive database on interprovincial heavy truck travel patterns in the National Capital Region. The Interprovincial Road Truck Survey is now available. Search about TRANSMandate The TRANS Committee was established in 1979 to coordinate efforts among major transport planning agencies. Copyright © 2021 · TRANS Committee ·

Genital Herpes Treatment Plan (Valacyclovir 500mg) - Roman
Valtrex - FDA prescribing information, side effects and uses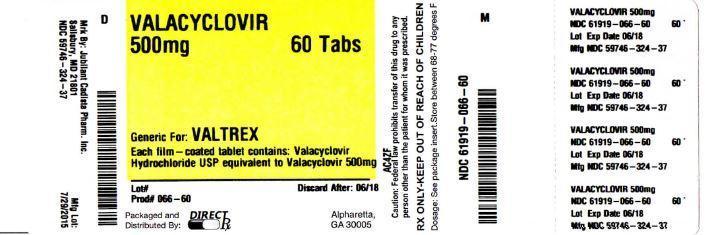
Valacyclovir
NDC 16714-698 Valacyclovir Hydrochloride Valacyclovir Hydrochloride
Valtrex Next Day FedEx Shipping, No Prescription Needed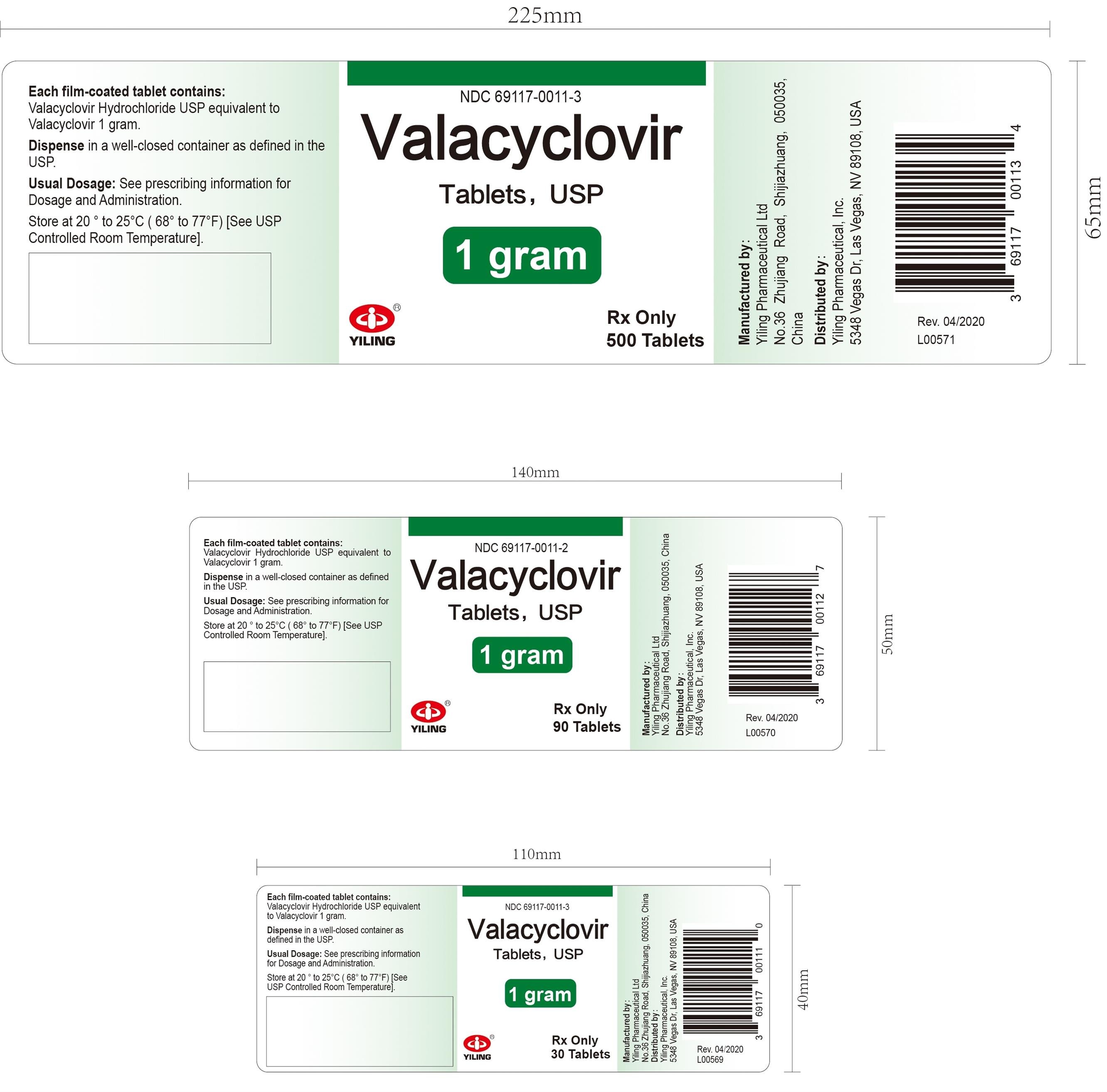
Valacyclovir - FDA prescribing information, side effects and uses
Valtrex side effects and interactions, and how to avoid them
Valacyclovir Genital Herpes Treatment | Prescribed Online | hims
What is Valtrex medication given for? - Quora
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ZOVIRAX Cream safely and
valacyclovir | Michigan Medicine
PDF) Acyclovir Nephrotoxicity: A Case Report Highlighting the Importance of Prevention, Detection, and Treatment of Acyclovir-Induced Nephropathy
Valacyclovir - wikidoc
Valtrex Tablets - NPS MedicineWise
Reference ID: 3481551
acyclovir (oral) | Cigna
Valtrex discount coupon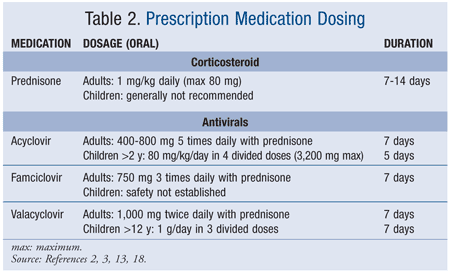
Bell's Palsy: To Treat or Not to Treat
Valacyclovir to buy uk — safe over the internet
COSMETIC LASER CENTER OF IRVINE
Are you a good candidate for Laser Hair Removal? Have you plucked, tweezed, waxed, bleached, or had electrolysis in the last 6 w
FRAXEL™ LASER CLINIC
How often should i take valacyclovir for cold sores, how often should i take valacyclovir for cold sores - No prescription TP24
Acyclovir Need Prescription – Healthdirect 24hr 7 days a week hotline
valacyclovir | Michigan Medicine
Valaciclovir – a first line antiviral medicine - BPJ 74 March 2016
Where To Order Valtrex Online Safe. Valacyclovir Generic Canada
Can You Get a Valtrex Prescription Online? - PlushCare
Valtrex (Valacyclovir Hydrochloride): Uses, Dosage, Side Effects, Interactions, Warning
Uses and Safety of Acyclovir in Pregnancy
Get Valacyclovir (Generic Valtrex) Online, if Prescribed - Rory
Valacyclovir | Side Effects, Dosage, Uses, and More
PDF) Immediate allergy from valacyclovir
Acyclovir Lovir 800mg Tablet- Uses, Dosage, Side Effects, Price, Benefits, Online Pharmacy - DoctorOnCall
Buy generic Acyclovir (herpes treatment) online
Valacyclovir for Shingles: How Effective Is It? - Roman HealthGuide
Famvir Next Day FedEx Shipping, No Prescription Needed
Valtrex Tablets - NPS MedicineWise![Search Results for]()
Search Results for "Valtrex No ⚰⤍📕 Buy Valtrex (Valacyclovir) from $2.55 per tab – 💜 www.LloydsPharmacy.online 💜 – Cheapest tabs 📕⤍⚰ Prior Prescription Buy Valtrex Overnight Valtrex Price Ph Where Can You
 Valtrex Tablets - NPS MedicineWise
Valtrex Tablets - NPS MedicineWise



























Posting Komentar untuk "valtrex no prior prescription"